 Is fluid restriction needed in heart failure? - Medwave
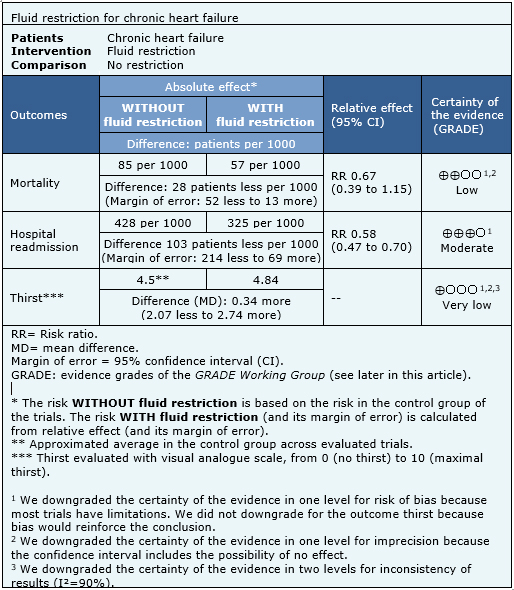
Is fluid restriction needed in heart failure? - MedwaveWarning: The NCBI website requires JavaScript to operate. Fluid management in patients with chronic heart failureAbstractCongestion or fluid overload, is a classic clinical characteristic of patients with heart failure and their presence is associated with adverse results. However, congestion is not always clinically evident, and more objective congestion measures than simple clinical examination can be useful. Although diuretics are the pillar of treatment for congestion, no randomized trial has shown the effects of diuretics on mortality in patients with chronic heart failure. Moreover, the proper rate of diuretics in this population is not clear. Research is required to determine whether a robust method of detection – and then treatment – subclinical congestion improves the results. Heart failure (HF) is one of the most common reasons for admission to the hospital. It is associated with long stays in patients, and has high morbidity and hospital and post-discharge mortality, if the left ventricular ejection fraction (VF) is reduced (FFHF) or normal (FNEF).[,] Congestion, or fluid overload, is a classic clinical characteristic of patients who present HF. In some patients, pulmonary congestion develops very quickly due to a sudden increase in VL filling pressures, and a precipitating factor is often recognized, such as acute myocardial ischaemia or uncontrolled hypertension. In this circumstance, edema is predominantly located in pulmonary airspace (pulmonary edema), while the total amount of fluid in the cardiovascular system remains unchangeable.[] However, for most patients, congestion is a more generalized process that is usually developed more gradually (experimental edema), and its management will be the center of discussion in this review. The build-up of chronic fluid is responsible for a substantial number of hospital income, and identifies patients with a worse prognosis than those admitted due to a sudden increase in VV filling pressures.[] Peripheral congestion in patients with heart failure is usually developed for weeks or even months, and patients may have "pleasantly" gained more than 20 liters of excess fluid, and therefore more than 20 kg of excess weight. The goal of management is to remove excess fluid, so that the patient no longer manages when leaving the hospital, now transferring to a diagnosis of "chronic HF (CHF)". However, for many patients, a certain degree of congestion remains even with treatment,[,] and it is not clear how many patients with ICC have subclinical congestion, that is, they have an excess of body fluid that does not reach the required volume to cause peripheral edema. Why do patients with heart failure have fluid? The development of peripheral edema in patients with IC is related to excess fluid. As the heart begins to fail, renal perfusion falls. The kidneys respond by increasing the production of renin, which leads to more aldosterone production, which is followed by the retention of sodium and water.[] Arginine vasopressin (AVP) is also released,[,] further improves fluid retention and stimulates thirst. The activation of the renin-angiotensin-aldosterone and AVP systems maintains preload (more fluid) and postload (vasoconstriction, mainly due to angiotensin II), thus maintaining the homeostasis of the cardiovascular system, but at a cost of increased systemic venous pressure (VP). The heart itself tends to worsen over time, as the failed VV tends to dilate, as does the left atrium, especially if the mitral regurgitation is developed. High PV can further reduce the flow of renal blood such as gradient between average renal blood pressure (often decreased by HF process) and PV decreases. The glomerular filtration rate falls, improving and perpetuating the vicious cycle. How do we identify congestion? The accumulation of liquids is a gradual process. In normal circulation, there is continuous filtration of fluid from intravascular space to tissues at a gradient-dependent rate between intravascular and extravascular hydrostatic pressure. Any filtered liquid is drained by lymphatic. Overloaded cardiogen peripheral edema develops because fluid retention causes increased intravascular hydrostatic pressure and a proportional increase in the filtration rate, which eventually exceeds the ability of lymphatic to drain the liquid (see ). HeFNEF = heart failure with normal ejection fraction; HFREF = heart failure with reduced ejection fraction; LV = left ventricle. Some patients do not present until they have developed generalized peripheral edema. In such cases the need for medical intervention is obvious. However, a substantial number of cases of subclinical congestion will not be clinically recognized, despite the presence of symptoms (i.e. inhalation). In patients without known heart disease, particularly in older people, the identification of subclinical congestion (and underlying heart dysfunction) at an earlier stage could change the path of the disease. In patients who are already known to have CF, if subclinical congestion is important is not clear. It used to be said that the evaluation by an experienced clinician is probably adequate to determine the fluid state.[] However, the art of the clinical examination is diminishing, partly due to the widespread availability of echocardiography and other functional or biochemical examinations, partly because the exact assessment may take a long time, especially in patients with poor mobility, and partly because clinical signs are not often specific to the disease; all of this leads to less qualified doctors in the clinical evaluation.[] On the other hand, doctors often disagree when faced with typical IC signs, which may have a unreliable relationship with diagnostic findings, including chest X-rays.[] However, a well done clinical examination in patients suspected of IC remains a powerful tool to identify patients with a worse prognosis, regardless of their LVEF. []The most reliable clinical sign indicating volume overloading is high jugular venous pressure (JVP), which also provides powerful prognostic information.[] However, the clinical evaluation of the JVP is often challenging and subjective[] and its ultrasound evaluation could be useful. In most cases, it is possible to evaluate the ugular vein by ultrasound and to identify patients with more advanced congestion and higher natriuretic peptides (PN),[] that are at increased risk of adverse results.[] The assessment of the lower diameter of the vena cava by echocardiography provides supplementary information to the clinical examination, is validated against invasively measured hemodynamics and is readily available in the echocardiographic departments.[,] The use of NPP as a measure of heart dysfunction is advised by the current guidelines. NPs are one of the defenses of the body against congestion.[] Any stretching of the myocardial leads to an increase in the NP level, and the elevated levels in a treated patient suggest that there is residual congestion, regardless of the LVEF. It is important to remember that circulating plasma levels should be adjusted for age and that comorbidities, including atrial fibrillation, kidney dysfunction and obesity, may influence NP levels.[] In clinical settings without easy access to echocardiography, measuring the NP level is a simple and reliable, efficient tool in terms of patient and personal time. NP evaluation at home is feasible with a finger test and this approach in high-risk patients can detect possible early decompensation. [] Invasive devices have potential as tools to predict congestion.[] Possible variables that can be measured include transthoracic impedance, pulmonary arterial pressure and left atrial pressure (LA). Implanable devices, such as pacemakers and/or defibrillators of cardiac resynchronization therapy (CRT) can measure intrathoracic impedance, thus estimating lung congestion, but its predictive value remains uncertain.[] In the HOMEOSTASIS trial, LA pressure measurements were taken twice a day in 40 patients with advanced IC. After the first 3 months, the treatment was personalised on the basis of readings, which led to a drop in the LA pressure (17.6 mmHg in the first 3 months to 14.8 mmHg; P =0.003), the highest dose prescription of angiotensin conversion inzima (ACE-I) and beta blockers, a lower need for high doses of diuretics of the New York stroke. [] In the CHAMPION trial, pulmonary blood pressure was measured daily using permanently implanted wireless devices in the pulmonary artery. There was a decrease of 37 per cent of the number of hospitalizations per IC during the 15 months of follow-up.[] An objective measure of 'congestion' could be useful not only to allow doctors to adapt treatments to specific goals, but also to allow more reproducible randomized controlled trials designed to evaluate decongestive strategies, especially when cardiac dysfunction markers (such as AMF) are not considerably impaired. For example, ALDO-DHF and TOPCAT trials in patients with HeFNEF suggested that while spironolactone could worsen renal function and anemia in patients with low circulation NPPs (and therefore presumably low congestion), it significantly reduced HF hospitalizations in patients with N-terminal superior prohormone cerebral natriuretic peptide.[NTBNP-promona] Most HF trials currently include high PNs as the main criterion for enrollment. Why does congestion matter? Congestion is an important cause of symptoms in patients with IC. The discomfort of the swollen legs and ascites precipitates hospitalization. Congestion is associated with the sensation of inhalation, especially when patients develop lung edema and pleural effusions. Congestion reduces liver function, and congested liver can be a source of discomfort. As described above, congestion causes renal dysfunction by reducing the gradient of transreal pressure. Anemia, which is very common among patients with IC, may worsen by congestion through dilution, and may exacerbate symptoms and heart dysfunction. [] The most common cause of hospitalization in patients with ICC is fluid retention and congestion.[] Hospitalization itself is associated with an adverse prognosis, and repeated hospitalizations are associated with an increasingly poor survival.[] Congestion itself, and not only reduced heart function, therefore it seems to be associated with a poor prognosis. Congestion is a powerful marker of an adverse prognosis and therefore is potentially an important therapeutic goal. How do we treat congestion in patients with chronic heart failure? Inducing a DiuresisDiuretics are the main pillar of management for patients with congestion. It has become a truism to assert that its use is based on empirical judgment and subjective clinical evaluation, rather than evidence-based medicine. Different kinds of diuretics are used in patients with chronic CF, although diuretics of loop (furosemide, bumetanide and torasemide) are the most prescribed. They exert their effect mainly by inhibiting the sodium-potassic-chloride cotransporter in the thick ascending extremity of the Henle Loop, avoiding the reabsorption of these ions, a posterior diuresis occurs. The diuretics of the loop mediate their effect from the luminal side of the tubular, so it is essential a glomerular filtration to allow them to work. The beneficial effects of a diuretic loop in JVP, lung congestion, peripheral edema and body weight have been known for years; diuretics also improve heart function, symptoms, and exercise tolerance in patients with IC.[] However, no randomized prospective study has ever assessed its impact on the outcome of chronic IC patients. Particularly in patients with severe kidney dysfunction, a reduced response is often observed and its use alone can be insufficient. For those who respond badly to a diuretic loop alone, the combination with a diuretic (or similar to tiazide) tizazide can be very powerful. Although the metolazone is often used in this scenario, there is little evidence that it is superior to other agents, such as the blessedflumethiazide.[] The test experience of combining several diuretic classes is still limited to just over 300 patients enrolled in small and mechanistic studies. [] The antagonists of meneralocorticoid receptors (MRA) are, of course, also diuretic. Two large trials[,] have shown that adding spironolactone or eplerenone to standard treatment in symptomatic patients with reduced LVEF (either chronic or after a recent myocardial infarction) produces morbidity and mortality benefits. If beneficial effects are due to a reduction in congestion are not at all clear given the wide range of actions of the MRAs.[] The MRA dose used to induce a diuresis is usually much higher than that used to treat chronic HF. The clinical benefits observed after the introduction of diuretics of loops are offset by a more marked activation of the renin-angiotensin system.[] An important issue to take into account is therefore if adding a diuretic in patients who are not clinically congested has some benefit. It is not clear at all whether diuretics lead to improved exercise capacity and/or improved biochemical measures of subclinical congestion (including NAPAs). Francisco and colleagues showed that acute injection of a diuretic loop (furosemide 1.3 ± 0.6 standard deviation [SD] mg/kg body weight) in patients who are not congested can cause transient adverse haemodynamic effects, with an increase in filling pressures of VL and a drop in the volume of trazo, with restoration of better hemodynamic and neurohumoral variables only. Other reports suggest that the use of diuretics unnecessarily (when there is no evidence of congestion) for a longer period of time could decrease systolic and diastolic blood pressure and increase the circulating levels of renin compared to placebo.[] Retrospective studies have raised concerns about a possible harmful effect of long-term use of loop diuretics in patients with CCI, possibly caused by chronic and sustained negative neuroendo. However, it is also logical to think that patients with the most severe IC will be prescribed more diuretic loop, which would have been associated with the adverse result.[] The relationship between the diuretic dose and the result needs further clarification, but there is a general belief that achieving the lowest tolerated dose, or even a definitive withdrawal of the diuretics of loop, could be beneficial. A small number of studies have attempted to identify patients who could tolerate diuretic withdrawal. Perhaps a third of patients with IC can tolerate diuretic removal of loop, especially if FEVI is higher than 27% or the base dose of diuretics of loop is ≤40 mg furosemide/day;[] this proportion increases significantly in patients who are not clinically considered at high risk of deterioration[] and may reach a success of up to 90% to 3 months of follow-up when the LVEF is normal.[] Vaptans block the action of vasopressin in their receptors, leading to the loss of water alone without a natriuresis – an acuresis call. In 142 patients with severe symptoms of CF, and compared to placebo, a single intravenous dose of conivaptan (20 or 40 mg) significantly reduced hair lung pressure and right atrial pressure during the first hours after administration, also increasing urine output to a dose-dependent amount.[] However, in the EVEREST trial, the use of hopperptan was not associated with any changes in clinical outcomes in a population of more than 4,000 patients admitted to acute CF. The enthusiasm for the routine use of vaptans has decreased, but they could certainly be useful in patients with hyponatraemia. Gheorghiade and colleagues showed that the hopper is at different doses (30, 45 or 60 mg/day) for 25 days was effective in reducing edema, and also normalized serum sodium in hyponatromic patients compared to placebo. [ ] Vaptans may be better than a diuretic loop as standard care. In a recent study, 83 patients treated in optimal medical treatment, with severe IC and clinical congestion, were randomized to placebo, monotherapy with hopperptan 30 mg/day or furosemide 80 mg or both tolvaptan 30 mg and furosemide 80 mg once a day for 7 days after a period of 2 days of low sodium diet (2 mg/day). Tolvaptan, or tolvaptan more furosemide, were well tolerated and produced a similar increase in urine output, greater than furosemide or placebo, without affecting blood pressure or other electrolytes other than sodium, which increased (although within normal values). [] The role of vaptans in routine practice remains uncertain, but several trials are on the way. For the time being, the guidelines of the European Society of Cardiology (ESC) only recommend that tolvaptan be used for patients with acute IC and resistant hyponatraemia; in the United States, vasopressin antagonists have a class IIb recommendation for short treatment of acute IC with persistent severe congestion and hyponatremia, at risk of (or having) active cognitive symptoms. Other drug inhibitors are the first-line treatment for chronic IC patients with reduced systolic function, unless contraindicated. They have a wide range of effects including the promotion of diuresis and renal excretion of sodium, mainly blocking the effects of angiotensin II in the kidney and the mediated secretion of angiotensin aldosterone II. At the same time, ACE inhibitors reduce circulating blood volume and venous and blood pressures; in addition, they not only improve the consumption of peak oxygen, but also decrease the levels of NP plasma in symptomatic patients[] or asymptomatic patients.[] The effect of CCA inhibitors on NPPs is independent of co-administration of beta blockers.[] In a trial that precedes modern therapy, patients whose symptoms and congestion were well controlled could not maintain clinical stability for long periods in diuretics alone. The risk of clinical decompensation was reduced when diuretics were combined with digoxin or an ACE-I.[] The addition of a direct renin-inhibitor to an ACE-I could further improve diuresis and decrease NP levels,[] but if this translates into a better long-term result is not yet known; the results for the ongoing ATMOSPHERE test are expected soon. LCZ 696 combines angiotensin receptor blockage (with valsartan) and neprilysin inhibition, an enzyme that degrades NPs, with sacubitril. LCZ 696 decreases the risk of death and hospitalization by IC in patients with stable chronic IC compared to enalapril. The drug increases by circulating active NP, as demonstrated by the increase in urinary levels of the guanosine monophosphate by bicycle (GMP), the second messenger of the NP, suggesting that it may have some role in reducing congestion. [] It has long been known that digoxin is used alone in patients with severe congestion, especially those with atrial fibrillation, can cause a deep diuresis. The effect is presumably secondary to the improvement of hemodynamics induced both by the slowing of heart rate and by the positive inotropic effect of digoxin, but it seems that there is a modest direct renal effect of digoxin.[] Since the introduction of diuretic loop, digoxin is very rarely used only for its diuretic effects. Levosimendan causes vasodilation of the coronary arteries and vessels of systemic resistance, decreasing preload and subsequent load. Some recent reports suggest that short and intermittent intravenous levosimendan courses could decrease NPPs and possibly IC hospitalization.[,] Greater trials are being conducted, evaluating the effectiveness of this new approach (LAICA: and ELEVATE). Other Factors Long-term education of patients with IC is of fundamental importance, to emphasize adherence to medicines and to monitor symptoms that indicate progression of the disease. Some patients may remain congested only because they do not take their prescription medications. About 25% of patients have difficulties in maintaining their follow-up appointments or taking their medications,[] and this ratio increases over time after diagnosis.[] A substantial proportion of patients continue to smoke despite adverse diagnosis.[] Nurses, doctors and other members of a multidisciplinary team, including a pharmacist, can provide education to patients, increase their compliance and, more importantly, improve the quality of life, decrease the readmission rate and relieve the economic burden of this increasingly common disease. []During times of severe fluid retention, simple interventions, such as continuous rest, could increase diuresis and significantly reduce body weight compared to posturel rest; Although the fluid restriction is an intervention mentioned by current guidelines for patients with IC, a recent metaanalysis, including five studies suggest that this therapy has no benefit compared to liberal fluid intake on mortality, admission or thirst in patients. [Course studies] The role of sodium restriction is not clear, although part of the traditional management of HF and recommended in the guidelines (although with a degree of evidence recognized low to support recommendations).[] In acute IC and congestion, the only effect of sodium restriction seems to be to increase the sensation of thirst.[] In patients with chronic IC, a normal sodium diet is associated with better results, although in the very high diuretic dose fund.[] It seems reasonable to suggest to patients that they should not add large amounts of salt to their diet, but excessive restriction has no role. The latest technologies, such as home supervision, can be used to educate patients beyond, but also to allow health professionals to monitor the symptoms and physiological variables of patients. The first telemonitoring studies suggest that it was useful, but the most recent studies are less compelling, perhaps because the "standard" trial extremity has improved significantly. [] Treatment of congestion in heart failure and normal ejection fracture In patients with IC and HeFNEF, there is no clear evidence that ACE inhibitors affect NP levels.[] In a study that enrolled 150 patients with HeFNEF randomized to diuretics alone (either furosemide or thiazides, depending on the level of congestion) or diuretics more irbesartan or ramipril, all treatments improved quality of life quickly (after 12 weeks). Plasma NT-proBNP fell after 12 months in the three groups, but the fall only reached statistical significance in the Ibrasartan (–124 (SD 302) pg/ml; P=0.01) and ramipril (-173 (SD 415) pg/ml; P=0.03) groups, suggesting a synergistic effect between the inhibitors of the renine-angiotensin system and diur. [] In patients with ICNEF, and compared to valsartan, LCZ 696 was effective in reducing NT-proBNP after 12 weeks of treatment in the PARAMOUNT trial, although the difference between treatment groups was no longer significant after 36 weeks of follow-up.[] If this is translated into results benefits, it is currently under investigation in the great PARAGON trial, which must be completed in 2019. Ultrafiltration and Abdominal Paracentesis In the most severe cases of HF, renal dysfunction and diuretic resistance often occur, and limit the therapeutic resources available to decrease congestion. While ultrafiltration is a generally reserved invasive solution for patients with severe acute IC, peritoneal dialysis (PD) is a home-based, intermittent and intermittent therapeutic option in which the removal of excess fluid is produced using peritoneum as a filter. Two recent studies of patients with advanced IC complicated by kidney failure have reported that PD is feasible, and that it could decrease body weight, and improve symptoms and functional status.[,] A recent systematic review of 21 studies (n=673 patients) suggests that PD improves ventricular function and decreases the number of days spent in the hospital with little risk of peritonitis (14 %/year).[]ConclusionsCongestion is a cardinal clinical characteristic of chronic HF and is linked to adverse results. Although several interventions could improve congestion, it is often not diagnosed. Little is known about the effects of anticongestive drugs par excellence, diuretics, on difficult results measures, such as mortality. The most objective measures of congestion (such as high NPPs, or ultrasound dilated vena cava) could allow the identification of patients at higher risk: trials are needed to test strategies for therapeutic decongestion in order to determine whether diuretic doses of loop, combined diuretic or more aggressive interventions such as peritoneal dialysis could improve the clinical state, possibly. ReferencesFormats: Share , 8600 Rockville Pike, Bethesda MD, 20894 USA
IMAGES

Fluid Restriction - Ted Rogers Heart Failure Patient Education
Fluid Restriction - Ted Rogers Heart Failure Patient Education
PDF) Fluid restriction in patients with heart failure: How should we think?
Perceptions of fluid restriction self-care in heart failure | British Journal of Cardiac Nursing
Non-pharmacological management of patients hospitalized with heart failure at a teaching hospital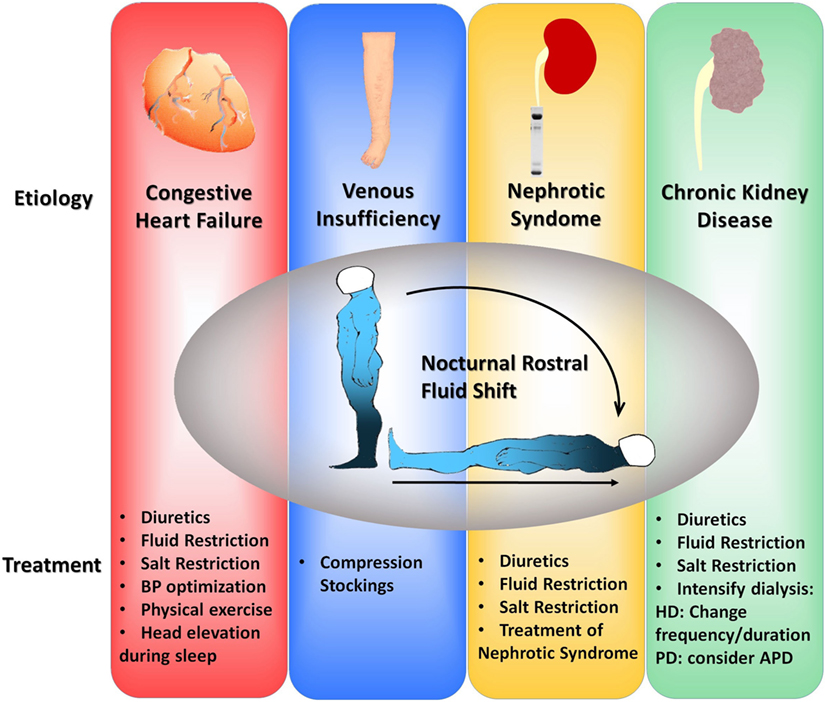
Frontiers | Fluid Redistribution in Sleep Apnea: Therapeutic Implications in Edematous States | Medicine
Patient Self-management in Chronic Heart Failure – Establishing Concordance Between Guidelines and Practice | CFR Journal
Healthy Living with Heart Failure: Your Nutrition matters - Quiz - YouTube
An app physicians should consider recommending for CHF and renal patients - iMedicalApps
Congestive Heart Failure in Canines | Today's Veterinary Practice
Cardiogenic shock & severe CHF - EMCrit Project
Nutrition Guide for Heart Failure - University of Ottawa Heart Institute
fluid restriction | Semantic Scholar
Untitled
Managing Heart Failure Symptoms | American Heart Association
Taking Control of Your Heart Failure (Patient Self-Care Book)
Congestive Heart Failure in Children
Salt Restriction in Heart Failure: Mind the Gap Segment | Core IM Podcast
Data Analysis and Research Design Study on Congestive Heart Failure
Solved: Part A -Calculating Fluid Intake For A Patient Wit... | Chegg.com
Decongestive therapy in heart failure: factors involved in the... | Download Scientific Diagram
Dietary Sodium Intake in Heart Failure | Circulation
Hypertension, Stroke and Congestive Heart Failure Lecture 7b Chapter 20 Dudek. - ppt download
Heart Failure management in long term care - ppt download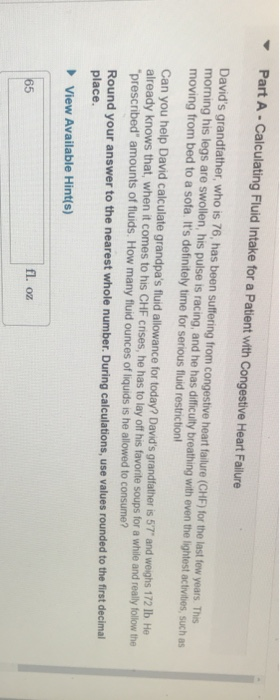
Solved: Part A- Calculating Fluid Intake For A Patient Wit... | Chegg.com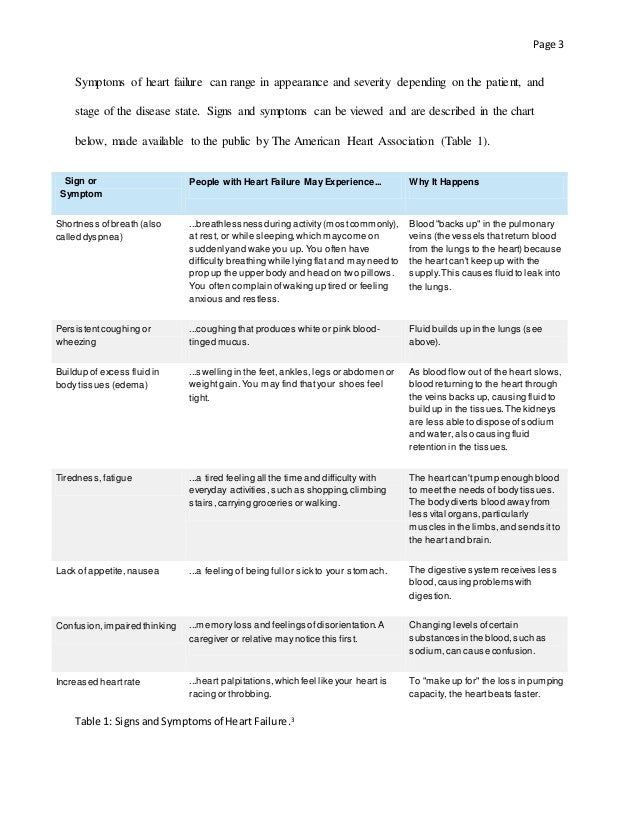
CHF Case Study
Non-pharmacological management of patients hospitalized with heart failure at a teaching hospital
Case Study | Symptomatic Hyponatremia in Congestive Heart Failure
Cardiogenic shock & severe CHF - EMCrit Project
CHF Patient Education
Data Analysis and Research Design Study on Congestive Heart Failure
Hyponatremia Treatment Algorithm CHF, Cirrhosis ... | GrepMed
Educational and Behavioral Content by Group Assignment | Download Table
Patient Self-management in Chronic Heart Failure – Establishing Concordance Between Guidelines and Practice | CFR Journal
Sodium Restriction - Ted Rogers Heart Failure Patient Education
2013 ACCF/AHA Guideline for the Management of Heart Failure | Circulation
Congestive Heart Failure in Canines | Today's Veterinary Practice
The prognosis of heart failure patients: Does sodium level play a significant role?
Salt and fluid restriction is effective in patients with chronic heart failure - Philipson - 2013 - European Journal of Heart Failure - Wiley Online Library
Nutrition Guide for Heart Failure - University of Ottawa Heart Institute
 Is fluid restriction needed in heart failure? - Medwave
Is fluid restriction needed in heart failure? - Medwave
































Posting Komentar untuk "fluid restriction for chf"